Chemistry
High Schoolers Beware: Do NOT Skip Chemistry!
BY: Ellen Mornague
It’s tempting, I know. But every time you walk out of the classroom, planning to never come back (assuming that you go the classroom at all), you miss loads of interesting facts, cool experiments, and the “Oh!” moment when you find out how every day things work. Not only do you need this knowledge to pass the course and find success in older grades, but you also miss out on a whole lot of fun!
So, have I convinced you to not skip class? No? Not yet? Well, sit back and relax, because I’m not done yet.
What exactly is in this course worth learning? I know that you think that class is just one lecture after another, one assignment after another, one test after another. But there’s more to it than that.
For students beginning grade 10 science, you usually start in the unit where experiments literally live: Chemistry. For those wanting the details, don’t worry; I hear you. I’ll give you a summary of the chemistry unit and all you need to know.
- Periods, Groups and Valence Electrons
Important Groups to Know:
- Alkali Metals EXCEPT HYDROGEN
- Group 1
- Have 1 valence electron
- Very Reactive
- Alkaline Earth Metals
- Group 2
- Have 2 valence electrons
- Less reactive than Alkali Metals & Halogens, but still reactive
- Halogens
- Group 17 (also known as Group 7)
- Have 1 valence electron
- Very Reactive
- Noble Gases
- Group 18 (also known as Group 8)
- Have no valence electrons
- Not reactive at all
Elements with the LEAST number of electrons to give away or take are the more reactive elements.
- Ions
Ions are atoms of an element that takes or gives away electrons to become a stable ion.
Positively charged ions = cations
Negatively charged ions = anions
Gaining electrons = anion
Losing electrons = cations
- Ionic And Molecular Compounds
- Ionic Compounds
Formed when ions of opposite charges combine by an ionic bond, because opposites attract. Literally.
Ionic compounds ALWAYS form between non-metals and metals.
For ionic compounds, the metal is usually the cation, and the non-metal is usually the anion. The non-metal will take electrons from the metal (because it is easier for a non-metal to take electrons rather than give them). This results in an ionic compound.
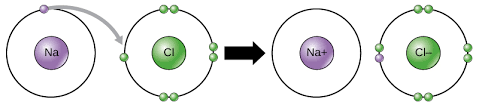
For example, sodium (Na) and chlorine (Cl):
Sodium’s valence electron joins chlorine’s valence electrons in the above picture, which is a perfect demonstration of ionic bonding.
See Figure 1
- Multivalent Metals / Transition Metals
Multivalent metals can form more than one (multiple) ions. The multivalent metals you learn about in grade 10 are:
- Copper
- Iron
- Tin
- Manganese
- Lead
When writing them, they are written with their charge next to them in Roman numerals and brackets. For example, a tin ion with a charge of four will be written as tin (IV).
- Polyatomic Ions
A polyatomic ion, like their name suggests, consist of more than one atom or element. For example, a carbonate ion is written as (CO3)2-, and it consists of carbon and oxygen.
In grade 10, you deal with 12 polyatomic atoms:
- Ammonium (NH4+)
- Phosphate (PO43-)
- Phosphate (PO33-)
- Carbonate (CO32-)
- Sulfate (SO42-)
- Sulfite (SO32-)
- Peroxide (O22-)
- Hydrogen carbonate (a.k.a bicarbonate) (HCO3–)
- Hydroxide (OH–)
- Nitrate (NO3–)
- Nitrite (NO2–)
- Chlorate (ClO3–)
- Acetate (C2H3O2–)
- Oxyacids
These acids are formed when hydrogen combines with a polyatomic ion. The polyatomic ion ends with the suffix “ic” and is followed by the word acid. Since acids are solutions, (aq) is always at the end (aq stands for aqueous, but that’s another matter [haha, get it? Matter and science…. you know what, never mind.])
These oxyacids are:
- Nitric acid (HNO3 (aq))
- Carbonic acid (H2CO3 (aq))
- Chloric acid (HClO3 (aq))
- Sulfuric acid (H2SO4 (aq))
- Phosphoric acid (H3PO4 (aq))
- Hydrochloric acid (HCl)
- Molecular Compounds
Molecular compounds are different from ionic because the atoms involved share each other’s electrons. When they share the electrons, the bond formed is called a covalent bond.
Naming these compounds are different from the others. Prefixes are used to indicate how many atoms of each element are in the compound.
The ones below are introduced to you in grade 10:
Prefix | Number |
Mono | 1 |
Di | 2 |
Tri | 3 |
Tetra | 4 |
Penta | 5 |
Hexa | 6 |
Hepta | 7 |
Octa | 8 |
Nona | 9 |
Deca | 10 |
- Chemical Reactions
- How to Write Equations in Chemistry
In a chemical reaction, there are two parts: the reactants and products. Let’s take aluminum oxide for an example:
Aluminum + Oxygen -> Aluminum Oxide
Things to notice in the equation:
- The reactants (in purple) are on the left side of the equation, and the products (in light blue) are on the left.
- The positions of the reactants and products in an equation are always the same!
- The “+” sign means “reacts with” or “and”, although you do not write those words in an equation.
- The arrow (in red) means “yields” or “produces”
The reaction Aluminum + Oxygen -> Aluminum Oxide is written verbally, or as a word equation. Another way to write it is by writing a chemical equation.
It would look something like this:
Al3 + O2 -> Al2O3
Less daunting, right?
When given the task to write an equation, you need to look carefully for the state of matter the substance is in. Why? Because at the end of the substance you need to write which state it is in.
This chart helps with that:
State | State symbol |
Solid | (s) |
Liquid | (l) |
Gaseous / Gas | (g) |
Aqueous (dissolves in water / soluble) | (aq) |
Let’s take this problem as an example:
When a piece of zinc metal is placed in a solution of copper (II) sulphate, a fuzzy reddish-brown coating of solid copper forms on the zinc. Highly soluble zinc sulfate is also produced, and energy is released.
The equations would be as follows:
Word equation:
Zinc + copper (II) sulphate -> copper + zinc sulfate + energy
Chemical equation:
Zn(s) + CuSO4(aq) -> Cu(s) + ZnSO4(aq) + energy
- Conservation of Mass
The Law of Conservation of Mass says that in a chemical reaction, the final mass of all the products equals the final mass of all the reactants.
This means that the equation needs to be equal on both sides.
How do we solve these types of equations?
Let’s use a sample problem.
If 2.9 g of hydrogen gas reacted with oxygen to produce 4.4 g of water, how much oxygen reacted with the hydrogen?
The chemical equation would be H2 (g) + O2 (g) -> H2O (l). And just like in math, we need to solve for the missing value, which is O2.
Since we are looking for the value of one of the reactants, we just subtract 4.4 (the mass of all products) from 2.9 (one of the values of the reactants) to get 1.5 g of oxygen, which has reacted to produce water.
- Balancing Equations
When there are not enough atoms of an element to equalize the equation, we balance it by adding coefficients in front of the incorrect formulas.
For example,
Na + O2 -> Na2O
The resulting balanced equation is 4Na + O2 -> 2Na2O
Once you do the math, the equation is balanced, as there is equal amounts of sodium and oxygen on both sides.
Note that if you can divide the coefficients by a common denominator, please do it. It makes the equation simpler.
- Types of Chemical Reactions
There are four chemical reactions (five if you count combustion).
Synthesis Reaction
When two or more simple reactants (usually elements) combine to make one complex product.
Pattern: A + B = AB
Example: 2Na + Cl2 -> 2NaCl
Decomposition Reaction
One large compound breaks down into two or more simple products.
Pattern: AB -> A+B
Example: 2AgCl -> 2Ag + Cl
Single Displacement Reaction
The element by itself kicks out the element in the compound.
Pattern: A + BC -> AC + B
Example: Al + NiBr3 -> Ni + AlBr3
Double Displacement Reaction
The cations in two compounds switch places.
Pattern: AB + CD -> AD + CB (A and C are cations, and B and D are anions)
Example: Pb (NO3)2 + KI -> K(NO3) + PbI2
- Acids and Bases
Acids are compounds that release hydrogen ions when it is dissolved in water. The formulas usually start with hydrogen.
Bases are compounds that release hydroxide ions (which look like OH–) when it is dissolved in water. The formulas have OH but does not start with it.
The chart below differentiates the properties of acids and bases:
Acids | Bases |
Sour Taste | Bitter taste |
Conduct Electricity | Conduct electricity |
Reacts with most metals and carbonates | Feels slippery |
Turns blue litmus red | Turns red litmus blue |
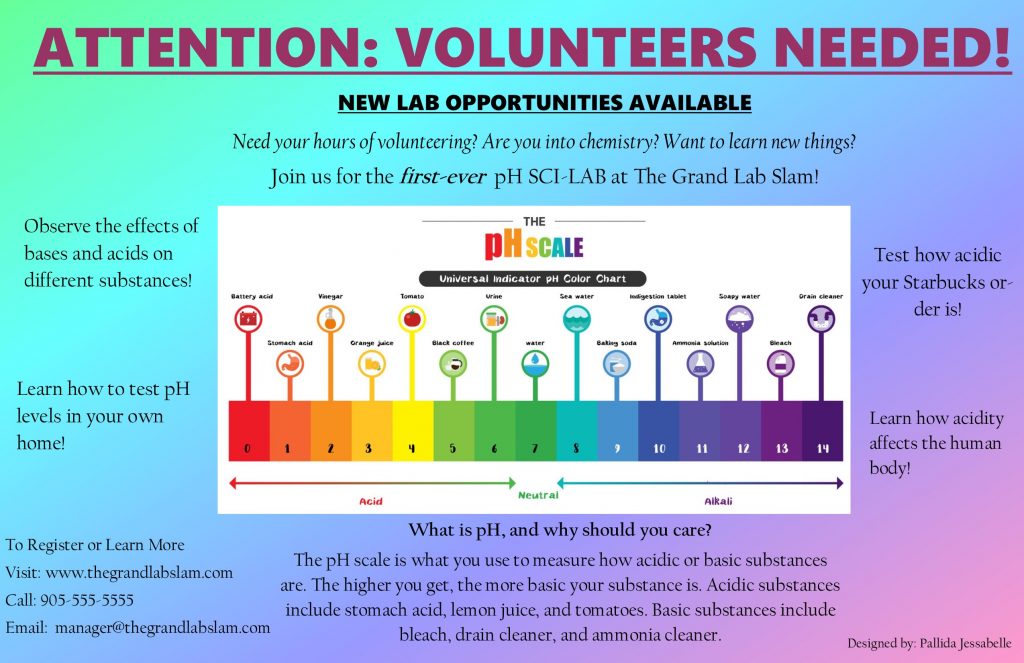
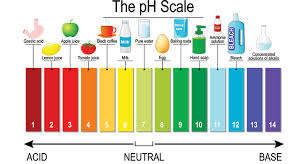
- The pH scale
The pH scale ranges from 0-14. If a substance falls in between 0-7, the substance is acidic or is an acid. If the substance falls in between 7-14, the substance is basic or is a base. The closer to 0 a substance is, the more acidic the substance is. The closer the substance is to 14, the more basic the substance is.
If a substance has a pH of 7, the substance is neutral.
See Figure 2
And there you have it! All the basics for chemistry. There is much more than this however, and it is taught in one place: the classroom. So, don’t skip. Trust me: you’ll lose out on a lot more than just quizzes. You’ll miss out on a whole new world.
References:
Notes in Class – Mrs. Rego

What’s in Your Air?
BY: Bernie Sanderson
You breathe it in, and you breathe it out. You drink a part of it, and your body transports it to keep you alive. Trees expel this. Does this substance sound familiar yet?
No? Well, it’s oxygen!
Oxygen is the eighth element on the Periodic Table of Elements, and it is essential to everyday life! Your respiratory system needs oxygen to perform the functions of your body, and to provide cells with energy. Water is made of one oxygen atom combined with two hydrogen atoms, and we need water to survive. There are many uses for oxygen, however.
Oxygen can be used in the medical field to treat disorders and diseases. Smelting iron requires a lot of oxygen to remove carbon from the iron and produce a fancy compound called ethylene glycol, which can transfer heat. There are even oxygen vending machines and oxygen bars, believed to cause a little euphoria.
But how did anyone know of oxygen? Who discovered it? How was it discovered?
In 1774 (that’s a long time ago!), Joseph Priestley and Carl Wilhelm Scheele discovered oxygen and called it an element. They did not do it together, but they discovered it around the same time. Scheele discovered it by heating substances like potassium nitrate and mercuric oxide and observed its reactions and properties. Priestley discovered oxygen by examining the thermal decomposition of mercuric oxide. Priestley published his findings first, which is why he is more commonly known as the discoverer of oxygen. But in reality, both Scheele and Priestley are the original discoverers. Antoine-Laurent Lavoisier, a chemist of French descent, unearthed the truth that oxygen enables humans to breathe, but he also discovered that oxygen can be used in combustion, which we will get into later.
Oxygen exists as a diatomic atom, which means that any time you see oxygen (with an incredibly good microscope, of course), it exists as two atoms, not one. Therefore, oxygen is written as O2. Other diatomic elements are Bromine (Br2), Iodine (I2), Nitrogen (N2), Fluorine (F2) Hydrogen (H2), and Chlorine (Cl2).
Oxygen has six valence electrons, which means that there are six extra electrons occupying the outer shell of the atom. However, for that atom to be stable, there needs to be eight electrons in the shell. Electrons don’t just form out of thin air; they need to either be shared, given away or taken. Sharing electrons creates either an ionic or molecular bond, which we will discuss later. Atoms will do what is easiest for them to do, or what takes the least amount of energy to do. If you have more electrons, atoms generally gain electrons; if you have less electrons, atoms generally lose electrons to eliminate the shell and make the atom stable. In this case, an oxygen atom has six valence electrons. Oxygen can either gain two more electrons or lose six electrons. Since gaining electrons is easier than losing them, oxygen gains two electrons and it becomes stable.
Oxygen is part of many reactions, but one of the most common reactions oxygen is associated with is the combustion reaction. This reaction happens when a fuel, oxygen and heat react together to release energy. This reaction is at its best when there is a lot of oxygen in the reaction, and a complete reaction brings more energy than an incomplete one, which has poor or less oxygen. An incomplete combustion can also create carbon monoxide, which is a dangerous and deadly chemical.
Oxygen is also part of chemical equations (yep, there are equations in chemistry!). For chemistry, equations need to be equal to work, otherwise it can lead to serious complications. Balancing equations can be hard, and it takes a lot of practice. But I care about you Sciencers, so I’ll give you an example.
Let’s start with the equation:
Na + O2 -> Na2O
The word equation will be:
Sodium + Oxygen -> Sodium Oxide
To balance it, you need to count all the atoms on the equation on both sides of the arrow. There is 1 sodium atom and 2 oxygen atoms on the left side of the arrow, and there is 2 sodium atoms and 1 oxygen atom on the right side of the atom.
Now that you know how many atoms are on either side, you need to make sure that there are the same number of element atoms on each side. The equation does not need to be balanced if the number are equal to begin with. Since this equation is unequal, we need to balance the equation. We do this by adding coefficients before the symbols.
Unfortunately, there is no one way of balancing equations, which is how it is hard.
I put a coefficient of 2 in front of the Na2O for starters. Then, because we needed 4 atoms of sodium, I put a coefficient of 4 in front of the Na on the left side. Once you do the math, it equals out. There will be 4 atoms of sodium on both sides, and 2 atoms of oxygen on both sides. It will look like this eventually:
4Na + O2 -> 2Na2O
This does not affect the written equation, however. The written equation will stay the same.
Oxygen is also part of a lot of bonds. One of them is a binary ionic bond. A binary ionic bond involves a metal and a non-metal. An example can be aluminum oxide (Chem Formula: Al2O3). Aluminum oxide is used for making dental cement. But how come there are 2 atoms of aluminum and 3 oxygen atoms?
As well, oxygen can bond with any element with 2 valence electrons. Why? Because while oxygen needs to gain 2 electrons to be stable (as stated earlier), elements with 2 valence electrons need to lose two in order to become stable. Since theses two elements need something from each other, they attract each other, stabilize and form an ionic bond with each other. For example, a stable magnesium atom has 2 valence electrons. In order to become stable, it needs to lose those electrons somehow. Coincidentally, oxygen has two spots open and available, because it needs to gain two electrons. Since the oxygen is negative (gaining more electrons makes the ion negative) and the magnesium is positive (losing more electrons makes the ion positive)
Oxygen is central to essential life and everything associated with life. Oxygen is the reason why our body works, how trees are healthy, and how we breathe. It’s sad that we don’t really take a breath and appreciate all the element does for us.
Here’s my challenge for you, Sciencers. Take a deep breath. Now, exhale, and silently thank oxygen for all the hard work it does.
References
https://azchemistry.com/common-uses-oxygen-element , November 5th, 2020
https://education.jlab.org/itselemental/ele008.html , November 5th, 2020
https://www.britannica.com/science/oxygen#ref279400 , November 5th, 2020
https://wiki.kidzsearch.com/wiki/Combustion , November 5th, 2020